Breast Implant Revision
Conveniently located to serve the areas of New York, NY
Breast implant revision surgery is a surgical procedure to address and correct issues or complications after a previous breast augmentation procedure.
Breast Implant revision surgery may include changes to existing breast implants and breast tissue to improve looks or to resolve some complications that can cause health issues.
Contents
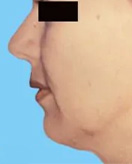
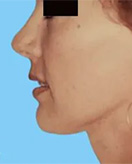
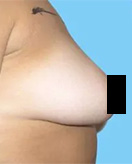
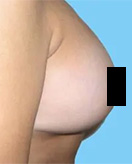
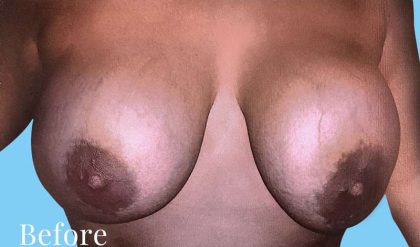
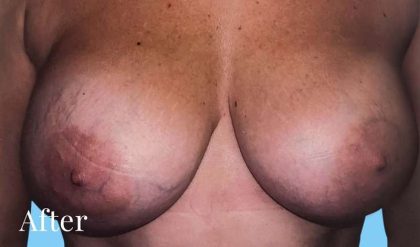
Before and After Photos
If you have had an implant rupture or are unsatisfied with the results performed by another plastic surgeon, Dr. Lefkovits can work with you to correct these problems.
You should know that breast implants change over time, and revisional surgery may be necessary for many reasons.
Reasons for Breast Revision Surgery
Breast revision surgery encompasses medical and cosmetic reasons, offering a range of factors that may prompt individuals to seek this procedure. Some of the main reasons for breast implant revision surgery are:
Implant rupture
Implant rupture is one of the most common reasons for revisional breast surgery, and it can be derived from implant age, trauma, or manufacturing defects. In this case implant revision is necessary because a damaged implant creates asymmetry of the breasts and may lead to health issues.
Implant Malposition
When breast implants move from their original position, it can result in asymmetry and unnatural-looking breasts. Breast revision is a way to correct the implant placement and back it to proper alignment.
Capsular Contracture
Breast revision in the case of capsular contracture includes removing or releasing the tightened scar tissue that becomes thickened and tightens around the implant. Capsular contracture can cause discomfort, breast shape distortion, and changes in implant position because scar tissue tightens around implants.

Aging and Tissue Changes
As we age, the overlying breast tissue naturally changes, such as decreased elasticity and sagging, which can impact the appearance of breast implants. Significant changes in weight, fluctuations, or changes in body composition can impact the appearance and position of breast implants. Weight gain or loss, pregnancy, or changes in muscle tone can cause the breasts to change shape and size, potentially affecting the implants.
Change in size or breast implants type
Over time our preferences change, and women often decide to adjust breast implant size to the desired volume and shape. The same situation occurs with breast implants type you may want to switch from saline implants to silicone implants or vice versa. In both cases, breast revision surgery allows for adjusting your breast implants size and change of the breast implants type.
Correcting Aesthetic Concerns and Dissatisfaction
You may be dissatisfied with the initial breast augmentation, or arise aesthetic concerns from previous surgery such as sagging and drooping, or change in shape, or you need to refine the position of the nipple-areola complex. Breast revision surgery may address these concerns and achieve the desired outcome.
Breast Revision Surgery Procedure
The breast revision surgery procedure may include several steps to address aesthetical unsatisfaction or complications associated with the previous breast augmentation surgery.
- Administration of anesthesia
Breast implant revision surgery is usually performed under anesthesia to ensure the patient’s comfort and safety. You can choose between two approaches general anesthesia, where the patient is asleep during the surgery, or local anesthesia with sedation, where you remain awake but relaxed.
- Incision Placement
Dr. Lefkovits will make incisions based on the predetermined surgical plan and the specific goals of the revision surgery. Whenever possible, he will try to use the previous surgical scar.
- Implant Replacement or Adjustment
Depending on the reasons for revision surgery, this step may include removing the existing implant or adjusting the implant pocket, reshaping the breast tissue, or utilizing techniques such as internal sutures to achieve symmetry and desired contour.
- Additional Procedures
Breast implant revision surgery offers an opportunity for additional procedures such as breast lift (mastopexy) to address sagging or drooping, fat grafting to enhance breast volume and contour, or nipple-areola complex adjustments to improve overall breast appearance. Breast revision plastic surgery may involve reshaping the breast implant pocket to achieve desired outcomes in breast augmentation revision by repositioning the implant.
- Incision Closure
In this step, the surgeon will close the incisions using sutures, adhesive skin closure, or surgical tape with minimal scars to ensure proper healing.
- Postoperative Care
The recovery process is crucial after any surgery, and the surgeon will provide you with all instructions on pain management, incision care, activity restrictions, and follow-up appointments.
Breast Implant Revision Surgery is a personalized procedure and may include some additional steps have in mind that this is the general outline of revision surgery.
Benefits
Breast implant revision surgery is beneficial for women undergoing primary breast augmentation procedures. Some of the key benefits of implant revision include:
- Enhanced Aesthetic Results
- Customized shape and size of the breast implants
- Resolve poor implant placement
- Improved breast implant position
- Address potential complications
- Increased self-confidence
- Opportunity to combine additional procedures such as mommy makeover
Risks
Breast implant revision surgery, like any plastic surgery, carries certain risks and potential complications. Possible risks may occur as bleeding and hematoma, infection, temporary or permanent changes in nipple or breast sensation, scarring, ruptured implant, or some aesthetic dissatisfaction.
It’s essential to be aware of these risks and discuss potential complications with our plastic surgeon during your consultation. Our qualified and experienced surgeon will take appropriate measures to minimize these risks and provide you with detailed information regarding the specific risks associated with your case. The plastic surgeon will also guide preoperative and postoperative care instructions to optimize your surgical outcome and reduce the likelihood of complications.
Choosing the right Surgeon
Finding a plastic surgeon you trust is crucial for breast implant revision surgery as it can be more challenging than the initial procedure. It is essential to select a skilled surgeon who can deliver high-quality results to achieve the desired outcome.
Our plastic surgeon can fix any of your previous breast procedures, including;
If your initial breast augmentation didn’t turn out the way you expected it to, Dr. Lefkovits can a breast revision to improve your breast size and shape. Dr. Lefkovits has been offering revisional breast surgery in New York, for over 30 years.
Dr. Lefkovits is a double board-certified plastic surgeon with extensive experience helping our patients feel more comfortable and confident with their appearance.
If you need revisional breast implant surgery, we can help We look forward to working with you.
If you have any questions, please contact our office at (212) 750-9494, and schedule a free consultation, or fill out the form on our website.
Our team would be more than happy to work with you to fit your unique needs.